Teacher Information
Current Location:Home 〉teacher
 Huimin YU, Professor
Huimin YU, ProfessorDepartment of Chemical Engineering, Tsinghua University
Key Laboratory of Industrial Biocatalysis, MOE, China
Center for Synthetic and Systems Biology, Tsinghua
Tel: 010-62795492
Email: yuhm@tsinghua.edu.cn
Lab:http://cbc.chemeng.tsinghua.edu.cn:8080/
http://www.chemeng.tsinghua.edu.cn/scholars/yuhm/yhm/indexen.html
Biography
Professor Yu’s research mainly focuses on Industrial biocatalysis, Synthetic biology and Bio-nanotechnology. Current efforts especially emphasize the fundamentals and applications of novel/ evolved biocatalysts via genome editing tools, promoter engineering, molecular chaperone engineering and metabolic engineering and bionanotechnology. Projects on “Smart Green Biomanufacturing”, such as developing a platform Rhodococcus cell-biocatalyst with high-solvent-tolerance, biomaufacturing valuable chemicals including acrylamide, biosurfactant, hyaluronic acid and chiral pharmaceutical intermediates, and designing novel bio-nano interdisciplinary systems such as Fe-Ni(Cu)@M13 micro electrolysis system and novel immobilized enzymes are highlighted. Currently, she is the associate editor of Biotechnology Notes, the editor of American Journal of Bioscience and Bioengineering, and Chemistry & Bioengineering. These works have harvested about 130 journal-publications and about 40 patents, and also obtained the supports from National Key R&D Program, 973 and 863 project, NSFC, National Top 100 PhD Theses Project, Beijing Nova (A) and multiple company collaboration projects, etc.
Education and Professional Career
1991-1996 B.S. in Chemical Engineering, Tsinghua University, China
1996-2001 Ph.D. in Biochemical Engineering, Tsinghua University, China
2000-2003 Assistant Professor in Tsinghua University
2005-2006 Visiting scholar in Massachusetts Institute of Technology, Boston, USA
2003-2012 Associate professor in Tsinghua University
2011-2016 Center head of Tsinghua University (ChemE Dept)-Shandong Polymer Company Biochemicals Joint center
2012-2016 Professor in Department of Chemical Engineering, Tsinghua University
2016-present Tenured Professor, Tsinghua University
Selected Awards
2017,Award for Technological Invention (2nd prize), Ministry of Education, China
2016,Award for Cooperation of Science and Technology in Dongying City, National Science and Technology Board of Dongying, Shandong Province.
2013, Award for Technological Invention (2nd prize), China Petroleum and Chemical Industry Association
2010, Outstanding Young Contributor in Science and Technology, China Petroleum and Chemical Industry Association
2008, New Century Excellent Talents in University, Ministry of Education
2007, Beijing Nova (A) in Science and Technology, Beijing Municipal Science & Technology Commission
2005, Hua-xin Prominent Scholar Award, Tsinghua University
2003, National Excellent Doctoral Dissertation
Research interests
Industrial biocatalysis; Synthetic biology;
Microbial metabolic engineering; Bio-nanotechnology
- Laboratory research highlight
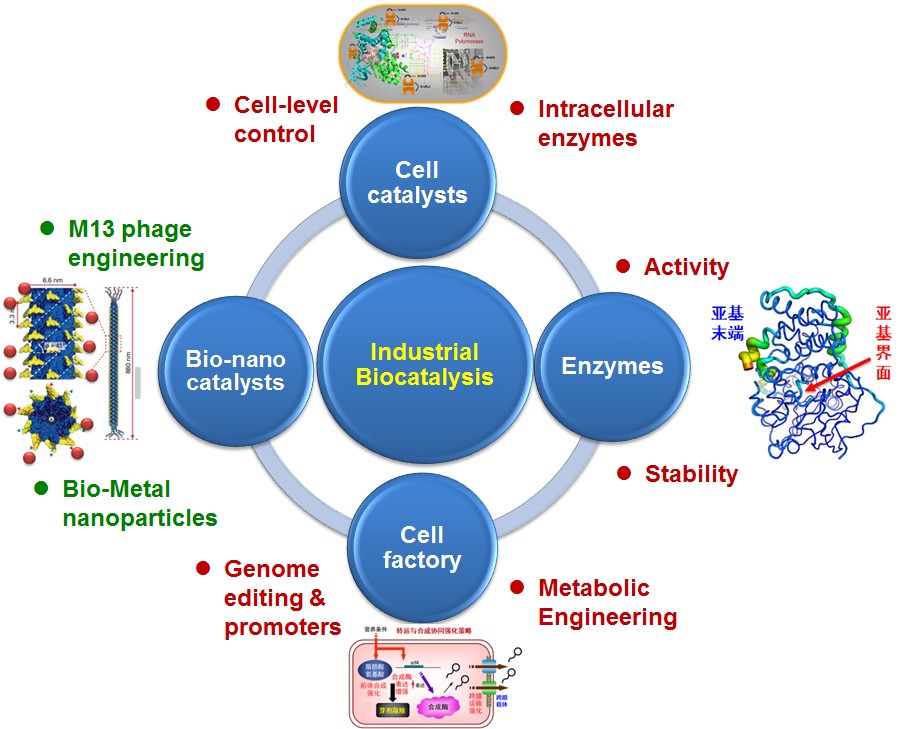
- Fundamentals of developing whole cell catalyst
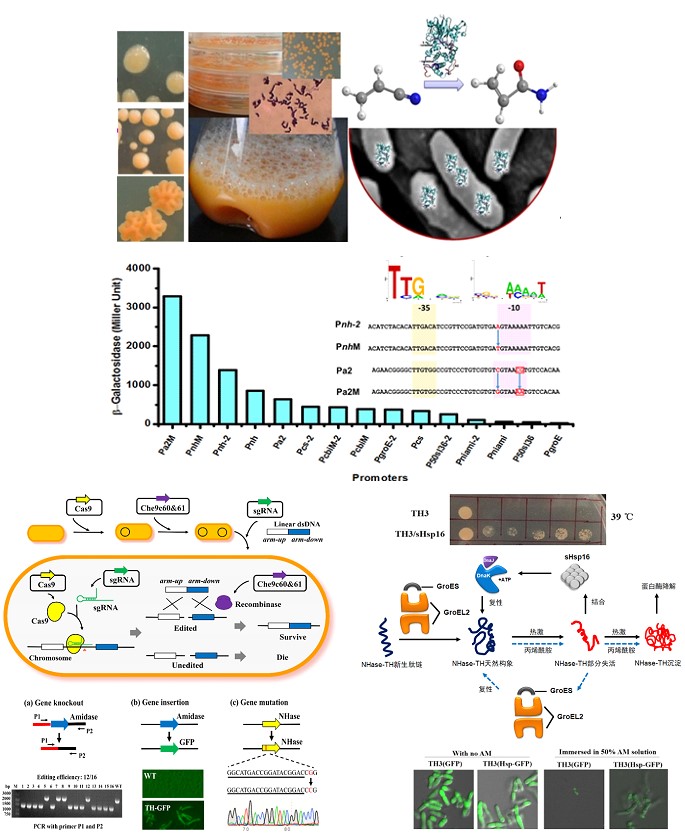
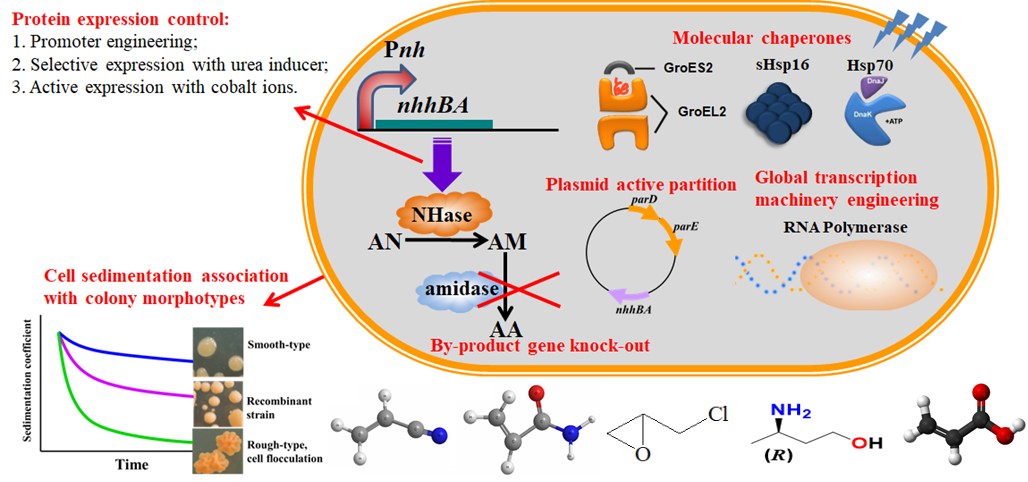
Rhodococcus cell biocatalysts, promoter engineering, Crispr/Cas9 genome editing tool and chaperones
- Enzyme engineering and whole cell catalysis
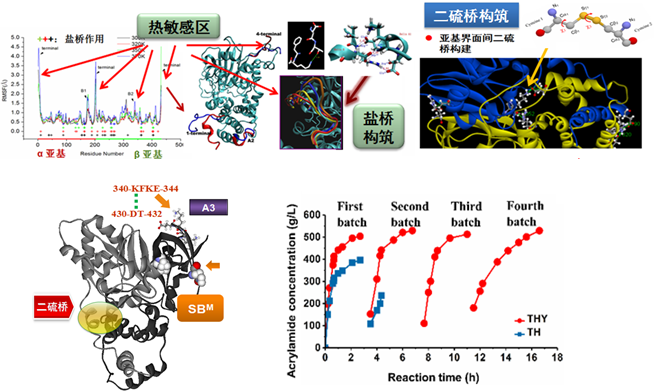
Designing salt-bridges and disulfide bonds in nitrile hydratase and the performance of the superior whole-cell Rhodococcus biocatalyst
- High-efficiency cell factory
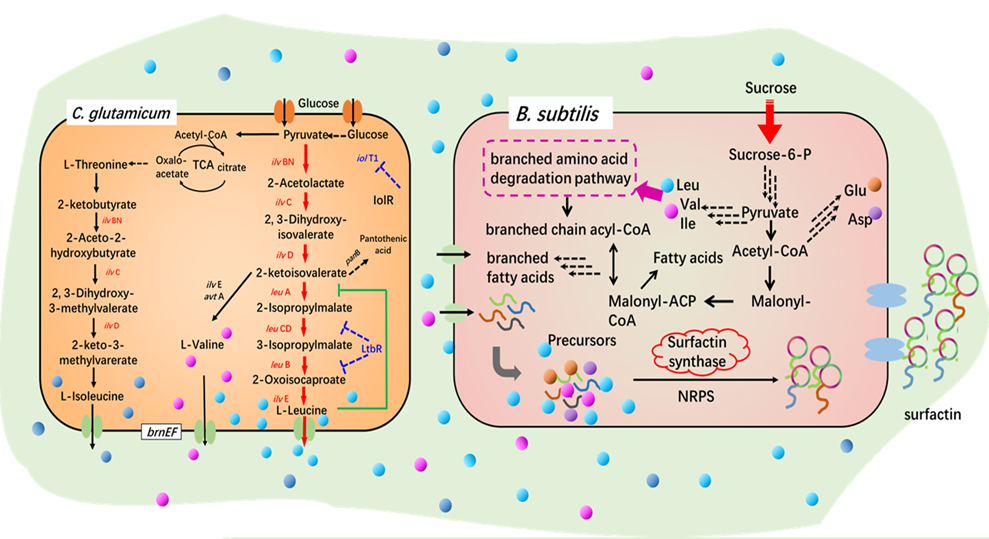
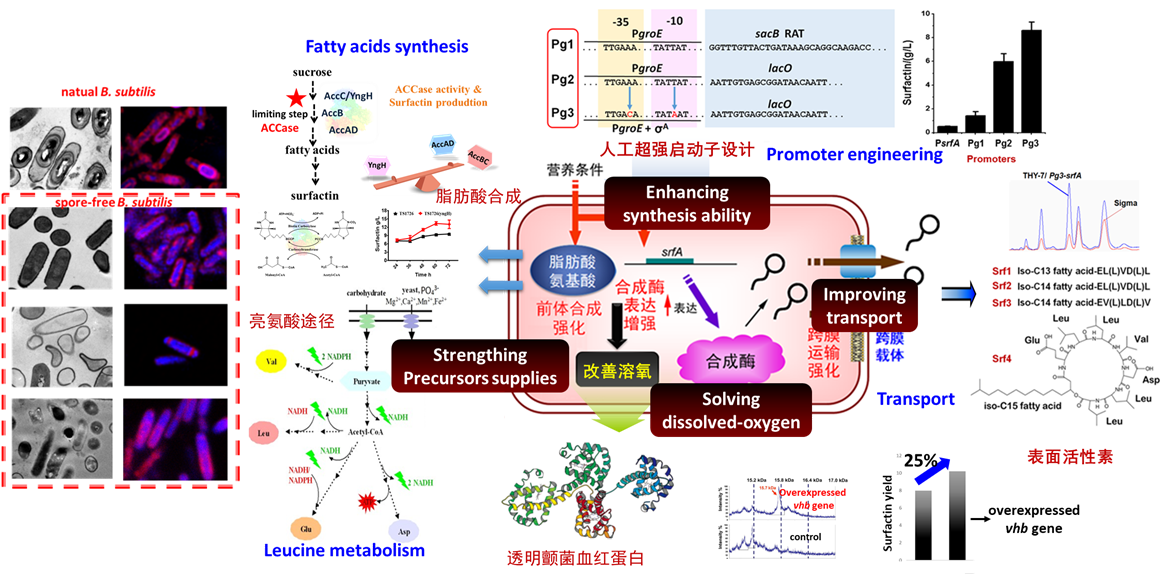
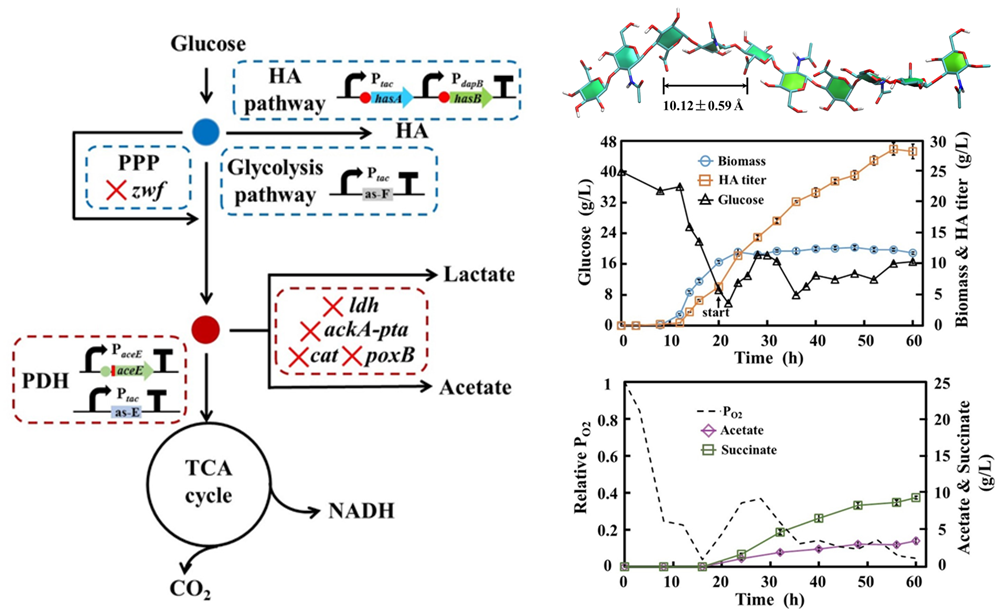
Bacillus subtilis and Corynebacterium glutamicum, surfactin and hyaluronic acid synthesis
- Nanobiotechnology
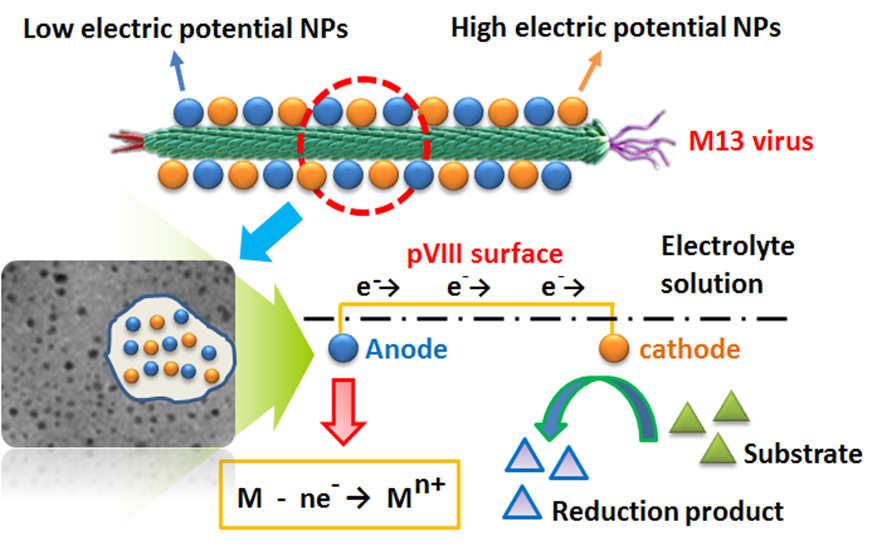
Fe-Ni@M13 micro-electrolysis system
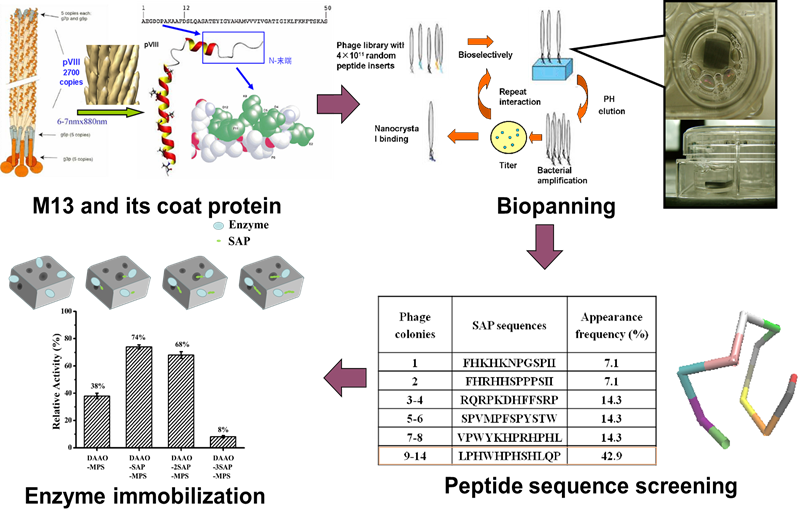
A novel immobilized enzyme method based on M13 phage
Selected publications
- Youxiang Liang, Song Jiao, Miaomiao Wang, Huimin Yu*, Zhongyao Shen. A CRISPR/Cas9-based genome editing system for Rhodococcus ruber TH. Metabolic Engineering, 2019, DOI: 10.1016/j.ymben.2019.10.003.
- Fangyu Cheng, HuiminYu*, Gregory Stephanopoulos*. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metabolic Engineering, 2019, 55:276-289.
- Miaomiao Wang, Huimin Yu*, Zhongyao Shen. Antisense RNA-based strategy to enhance ACCase activity and surfactin production in Bacillus subtilis TS1726. ACS synthetic biology. 2019, 8: 251-256.
- Lingjun Tang#, Ji Yang#, Jie Chen, Jing Zhang, Huimin Yu*, Zhongyao Shen. Design of salt-bridge cyclization peptide tags for stability and activity enhancement of enzymes. Process Biochemistry 2019, 81: 39-47.
- Youxiang Liang, Song Jiao, Miaomiao Wang, Huimin Yu*, Zhongyao Shen. Overexpression of epoxide hydrolase in Rhodococcus ruber with high robustness for the synthesis of chiral epichlorohydrin. Process Biochemistry. 2019, 79, 49-56.
- Miaomiao Wang#, Wenjing Qi#, Hongping Xu, Huimin Yu*. Affinity binding immobilization of D-amino acid oxidase on mesoporous silica by a silica-specific peptide. Journal of Industrial Microbiology & Biotechnology, 2019. 1-7.
- Song Jiao, Huimin Yu*, Zhongyao Shen. Core elements characterization of Rhodococcus promoters and development of a gradient intensity mini-pool for efficient gene expression. New Biotechnology, 2018, 44, 41-49.
- Miaomiao Wang*, Jie Chen, Huimin Yu*, Zhongyao Shen. Improving stress tolerance and cell integrity of Rhodococcus ruber by overexpressing small‑shock‑protein Hsp16 of Rhodococcus. Journal of Industrial Microbiology & Biotechnology (2018) 45:929–938.
- Yangzi Chen, Song Jiao, Miaomiao Wang, Jie Chen, Huimin Yu*. A novel molecular chaperone GroEL2 from Rhodococcus ruber and its fusion chimera with nitrile hydratase for co-enhanced activity and stability. Chemical Engineering Science, 192 (2018) 235–243.
- Fangyu Cheng, Sijin Luozhong, Zhigang Guo, Huimin Yu*, Gregory Stephanopoulos. Enhanced biosynthesis of hyaluronic acid with engineered Corynebacterium glutamicum via metabolic pathway regulation. Biotechnology Journal. 2017, 12, 1700191.
- Ji Yang, Fangyu Cheng, Huimin Yu*, Junting Wang, Zhigang Guo and Gregory Stephanopoulos. Key role of the carboxyl terminus of hyaluronan synthase in processive synthesis and size control of hyaluronic acid polymers. Biomacromolecules 2017, 18, 1064−1073.
- Song Jiao, Jie Chen, Huimin Yu*, Zhongyao Shen. Tuning and elucidation of the colony dimorphism in Rhodococcus ruber associated with cell flocculation in large scale fermentation. Appl Microbiol Biotechnol., 2017, 101:6321-6332.
- Song Jiao, Xu Li, Huimin Yu*, Huan Yang, Xue Li and Zhongyao Shen. In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters. Biotechnology and Bioengineering 2017, 114(4): 832–842.
- Shuai Zhang, Huimin Yu*, Ji Yang, Zhongyao Shen. Design of the nanoarray-pattern Fe-Ni bi-metal nanoparticles@M13 virus for enhanced reduction of p-chloronitrobenzene through micro-electrolysis effect. Environmental Science: Nano, 2017, 4, 876 - 885.
- Jizhe Sun, Huimin Yu*, Jie Chen, Hui Luo, Zhongyao Shen. Ammonium acrylate biomanufacturing by an engineered Rhodococcus ruber with nitrilase overexpression and double-knockout of nitrile hydratase and amidase. Journal of Industrial Microbiology & Biotechnology. 2016, 43(12):1631-1639.
- Huan Yang, Xu Li, Xue Li, Huimin Yu*, Zhongyao Shen. Identification of Lipopeptide Isoforms by Tandem MALDI-TOF-MS/MS Based on the Simultaneous Purification of Iturin, Fengycin and Surfactin by RP-HPLC. Analytical and Bioanalytical Chemistry. 2015, 407(9): 2529-2542.
- Xu Li, Huan Yang, Donglai Zhang, Xue Li, Huimin Yu*, Zhongyao Shen. Overexpression of specific proton motive force-dependent transporters facilitate the export of surfactin in Bacillus subtilis. Journal of Industrial Microbiology & Biotechnology: 2015, 42(1), 93-103.
- Jie Chen, Huimin Yu*, Changchun Liu, Jie Liu, Zhongyao Shen. Improving stability of nitrile hydratase by bridging the salt-bridges in specific thermal-sensitive regions. Journal of Biotechnology 164 (2013) 354–362
- Huimin Yu*, Keith Tyo, H. Alper, D. Marcuschamer, Gregory Stephanopoulos*. A High Throughput Screen for Hyaluronic Acid Accumulation in Recombinant Escherichia coli Transformed by Libraries of Engineered Sigma Factors. Biotechnology and Bioengineering. 2008, 101 (4): 788-796.
- Huimin Yu*, Gregory Stephanopoulos*. Metabolic Engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metabolic Engineering. 2008, 10(1): 24-32.



